- The Basics of Iron
- Physical Properties of Iron
- Chemical Properties of Iron
- Uses of Iron
- Safety Considerations
- Sources of Iron
- Benefits of Using Iron Sulfate
- 1. Soil and Plant Health
- 2. Weed Control
- 3. Water Treatment
- 4. Colorant
- 5. Metal Surface Treatment
- 6. Other Applications
- The Basics of Copper Sulfate
- What is Copper Sulfate?
- Properties and Characteristics
- Uses of Copper Sulfate
- Safety Considerations
- Benefits of Using Copper Sulfate
- 1. Effective Weed Control
- 2. Algae Control
- 3. Fungicide
- 4. Livestock Health
- 5. Snail and Parasite Control
- 6. Pool and Spa Maintenance
- 7. Ornamental Use
- 8. Industrial Applications
- 9. Affordable and Readily Available
- 10. Environmentally Friendly
- Differences between Iron and Copper Sulfate
- 1. Chemical Composition
- 2. Physical Appearance
- 3. Uses
- 4. Reactivity
- 5. Toxicity
- 6. Environmental Impact
- Conclusion
- When to Use Iron Sulfate
- 1. Treating Iron Deficiency
- 2. Correcting Soil pH Levels
- 3. Moss Control
- 4. Algae Control in Water
- 5. Fertilizer for Acid-Loving Plants
- When to Use Copper Sulfate
- 1. Algae and Weed Control
- 2. Fungicide
- 3. Animal Repellent
- 4. Cleaning and Sterilization
- 5. Copper Deficiency in Plants
- Precautions and Considerations
- Protective Clothing
- Proper Ventilation
- Storage
- Environmental Impact
- Dosage and Application
- Question-answer:
- What is iron sulfate?
- What is copper sulfate?
- When should I use iron sulfate?
- When should I use copper sulfate?
- Can I use iron sulfate instead of copper sulfate?
- What are the potential risks of using copper sulfate?
- Video: Chemistry Revision – Iron & Copper Sulphate solution
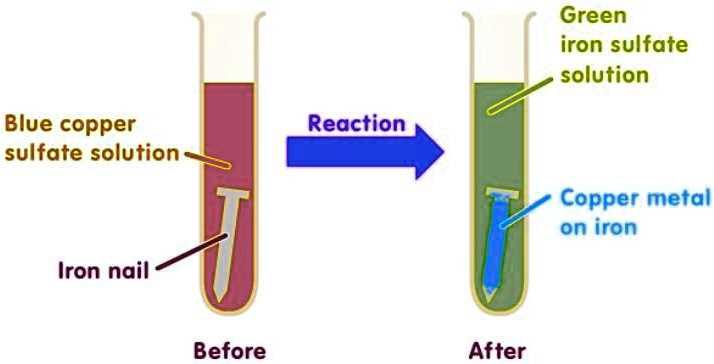
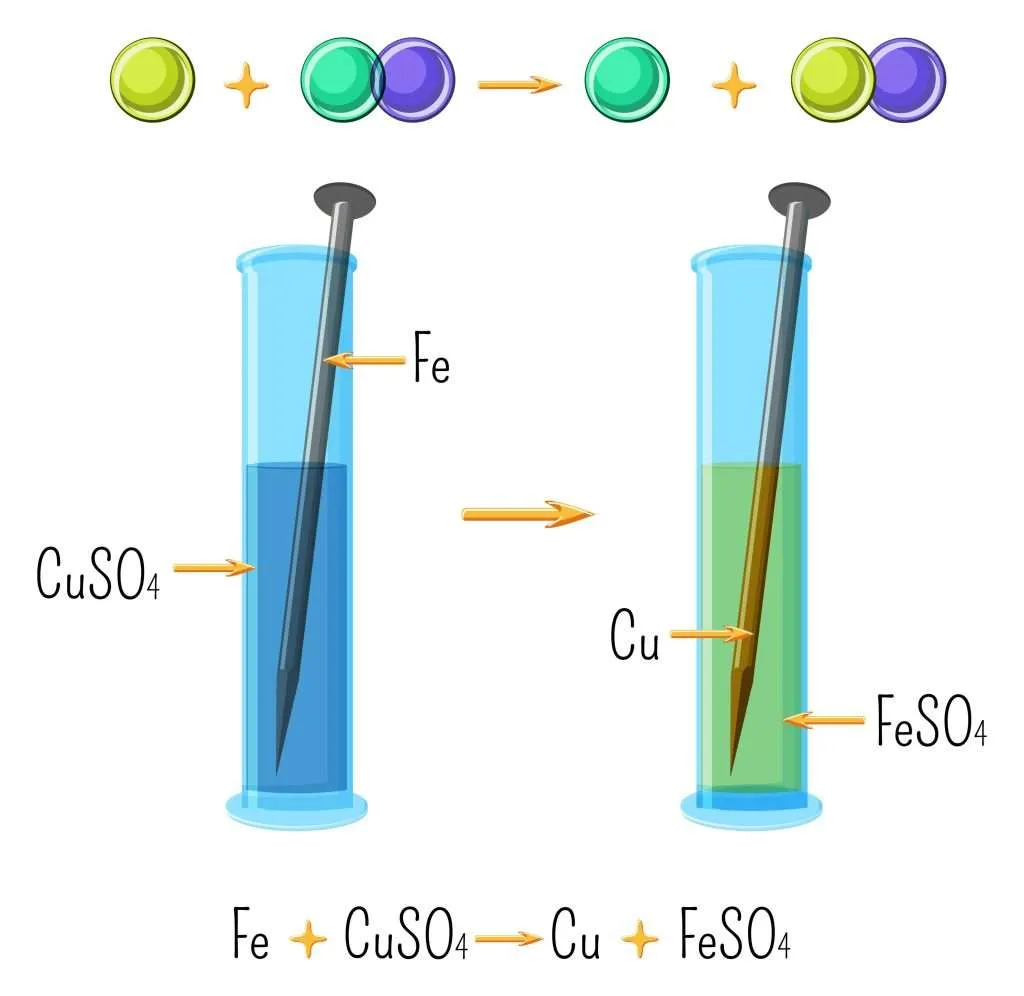
Iron and copper sulfate are two common chemicals that are often used in various applications. They each have their own unique properties and uses, and understanding when to use each one is important. In this article, we will delve into the differences between iron and copper sulfate, their various applications, and what you need to know about each chemical.
Iron is a metal that is known for its strength and durability. It is commonly found in construction materials, such as nails and screws, due to its ability to withstand significant amounts of pressure. Iron is also used in the production of steel, which is used in buildings, appliances, and vehicles. In addition, iron is an essential mineral for the human body and is crucial for the production of red blood cells. Iron supplements are often recommended for individuals with iron deficiency anemia.
Copper sulfate, on the other hand, is a chemical compound made up of copper, sulfur, and oxygen. It is commonly used as a fungicide and herbicide in agriculture to control plant diseases and unwanted plant growth. Copper sulfate is also used in various industries, including mining, metal plating, and printing. It is known for its blue color and is often used as a dye in textiles and inks.
When deciding whether to use iron or copper sulfate, it is important to consider the specific application. Iron is best suited for applications where strength and durability are key, such as construction and manufacturing. Copper sulfate, on the other hand, is ideal for agricultural and industrial applications where its fungicidal and herbicidal properties are needed. It is important to note that both iron and copper sulfate should be handled with care, as they can be toxic if ingested or inhaled. Proper safety precautions should always be taken when working with these chemicals.
The Basics of Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table.
Iron is one of the most abundant elements on Earth and is widely used in various industries due to its strength and durability. It has been used by humans for thousands of years, with evidence of iron artifacts dating back to around 3000 BCE.
Physical Properties of Iron
- Appearance: Iron is a silver-gray metal with a lustrous appearance.
- Melting Point: The melting point of iron is 1535 degrees Celsius (2795 degrees Fahrenheit).
- Boiling Point: The boiling point of iron is 2750 degrees Celsius (4982 degrees Fahrenheit).
- Density: The density of iron is 7.87 grams per cubic centimeter.
- Hardness: Iron is a relatively hard metal with a Mohs hardness of 4.
Chemical Properties of Iron


Iron is a reactive metal and readily oxidizes in the presence of moisture and oxygen. This is why iron objects, if not protected, tend to rust over time.
Iron can form various compounds, including oxides, sulfides, and alloys. It readily reacts with acids to form salts, such as iron(II) sulfate (FeSO4) or iron(III) chloride (FeCl3).
Uses of Iron
Iron is primarily used for the production of steel, which is an essential material in construction, infrastructure, and manufacturing. It is also used in the production of various alloys, such as cast iron, stainless steel, and wrought iron.
Other uses of iron include:
- Transportation: Iron is used in the manufacturing of automobiles, ships, trains, and aircraft.
- Tools and Machinery: Iron is used to make tools, machinery, and equipment for various industries.
- Home Appliances: Iron is used in the production of appliances like refrigerators, ovens, and washing machines.
- Electronics: Iron is used in the production of magnets and magnetic storage devices.
Safety Considerations
While iron is an essential element for living organisms, excessive levels of iron in the body can be toxic. Iron supplements should be used with caution and under the guidance of a healthcare professional.
When handling iron, it is important to take proper safety precautions, including wearing protective gloves and goggles to avoid injury.
Sources of Iron
Iron is found in abundance in the Earth’s crust and can be extracted from various ores, such as hematite and magnetite. It is also present in many food sources, including red meat, poultry, fish, beans, and leafy green vegetables.
It is worth noting that the availability of iron from plant-based sources is lower compared to animal-based sources. Consuming vitamin C-rich foods along with iron-rich foods can enhance the absorption of iron from plant sources.
Benefits of Using Iron Sulfate
Iron sulfate, also known as ferrous sulfate, is a commonly used compound in various industries and applications due to its many benefits. From agriculture to water treatment, iron sulfate offers numerous advantages that make it a go-to choice for different purposes.
1. Soil and Plant Health
One of the major benefits of using iron sulfate is its positive impact on soil and plant health. Iron is an essential nutrient for plants, and iron sulfate is a highly soluble form that is readily available for absorption by plants. Adding iron sulfate to soil can help correct iron deficiencies, improve overall plant growth and development, and increase crop yield.
2. Weed Control
Iron sulfate is often used as a herbicide to control and kill weeds. When applied to the leaves of unwanted plants, iron sulfate disrupts their normal metabolic processes and causes them to wither and die. This makes it an effective and environmentally friendly alternative to synthetic herbicides.
3. Water Treatment
Iron sulfate is commonly used in water treatment processes to remove impurities and improve water quality. It acts as a coagulant, helping to clump together particles and solids in water, making it easier to filter and remove them. Additionally, iron sulfate can also help control algae growth in ponds, lakes, and other water bodies.
4. Colorant
Iron sulfate can be used as a colorant in various applications. It is commonly utilized in the production of dyes, paints, and pigments, giving them a brown or yellow color. The use of iron sulfate as a colorant is cost-effective and provides a unique hue to many products.
5. Metal Surface Treatment
Iron sulfate is often used as a metal surface treatment for various purposes. It can be applied to steel or iron surfaces to provide a protective coating against rust and corrosion. This treatment helps extend the lifespan of metal structures and equipment, making them more durable and long-lasting.
6. Other Applications
In addition to the above benefits, iron sulfate has various other applications. It can be used in the production of fertilizers, animal feeds, and pharmaceuticals. Iron sulfate is also used in the textile and leather industry for dyeing and tanning processes.
Overall, iron sulfate offers a wide range of benefits in different industries and applications. Its versatility, affordability, and effectiveness make it a valuable compound for various purposes.
The Basics of Copper Sulfate
Copper sulfate is a commonly used chemical compound that is known for its diverse applications. It is the combination of copper and sulfuric acid, resulting in a blue crystalline solid. With its wide range of uses, understanding the basics of copper sulfate can be beneficial for various purposes.
What is Copper Sulfate?
Copper sulfate, also known as cupric sulfate, is a chemical compound with the formula CuSO4. It consists of copper, sulfur, and oxygen atoms and exists in different forms, such as anhydrous (without water) and hydrated (with water) crystals.
The most commonly used form is pentahydrate copper sulfate (CuSO4·5H2O), which is a bright blue crystalline solid. This form is widely available and can be easily dissolved in water, making it a popular choice for various applications.
Properties and Characteristics
Copper sulfate has several properties and characteristics that make it useful in different fields:
- Color: Copper sulfate is typically blue in color. This distinct color helps identify its presence in solutions or products.
- Solubility: It is highly soluble in water, allowing it to be easily dissolved and used for various purposes.
- Crystalline Structure: Copper sulfate forms crystals with a specific geometric arrangement. These crystals can be large or small, depending on the conditions of its formation.
- Odor: Copper sulfate has a faint odor, but it is generally not significant enough to cause any discomfort.
- Toxicity: While copper sulfate is toxic to certain organisms in high concentrations, it is relatively safe for humans to handle in the appropriate quantities and with proper precautions.
Uses of Copper Sulfate
Copper sulfate has a wide range of applications across various industries and fields:
- Agriculture: It is used as a fungicide and pesticide in agriculture to control the growth of fungi and algae. It can also be used to correct copper deficiencies in soil.
- Animal Husbandry: Copper sulfate is added to animal feed as a trace mineral supplement, promoting healthy growth and preventing deficiencies.
- Chemistry: It is used in various chemical reactions and laboratory experiments as a reagent or catalyst.
- Electroplating: Copper sulfate is used in electroplating processes to create a layer of copper on objects, providing them with a protective and decorative coating.
- Water Treatment: It is used in water treatment processes to control algae and bacterial growth in pools, ponds, and water systems.
- Art and Crafts: Copper sulfate can be used as a dye or patina to create a blue or greenish-blue color on various materials, including metals and fabrics.
Safety Considerations
While copper sulfate has numerous uses, it is essential to handle it with caution and follow safety guidelines:
- Wear appropriate protective equipment, such as gloves and goggles, when handling copper sulfate.
- Store copper sulfate in a cool, dry place away from incompatible materials.
- Follow proper disposal methods for copper sulfate to prevent harm to the environment.
- Avoid ingestion or inhalation of copper sulfate. In case of accidental exposure or poisoning, seek medical attention immediately.
| Common Name | Copper Sulfate |
|---|---|
| Chemical Formula | CuSO4 |
| Appearance | Bright blue crystalline solid |
| Solubility | Highly soluble in water |
| Uses | Agriculture, animal husbandry, chemistry, electroplating, water treatment, art and crafts |
Benefits of Using Copper Sulfate
1. Effective Weed Control
Copper sulfate is a powerful herbicide that can be used to control a wide range of weeds. When applied to the soil, it inhibits the germination and growth of weeds, helping to keep your garden or agricultural field free from unwanted plants.
2. Algae Control
Copper sulfate is also commonly used to control algae growth in bodies of water. It can be applied directly to ponds, lakes, and other water sources to eliminate algae blooms and prevent further growth. This is particularly beneficial for maintaining water quality and preventing the spread of harmful algal blooms.
3. Fungicide
Copper sulfate has fungicidal properties, making it an effective treatment for various fungal diseases in plants. It can be used to protect crops and ornamental plants from diseases such as blight, mildew, and leaf spot. Regular applications of copper sulfate can help maintain the health and productivity of your plants.
4. Livestock Health


Copper sulfate is commonly used as a nutritional supplement for livestock to promote growth and prevent copper deficiency. It can help improve the overall health and wellness of animals, leading to better productivity and profitability in livestock farming.
5. Snail and Parasite Control
Copper sulfate can effectively control snails and parasites in aquatic environments. It is commonly used in fish ponds and aquariums to prevent infestations and maintain the health of aquatic organisms. By eliminating these pests, copper sulfate helps maintain a balanced ecosystem.
6. Pool and Spa Maintenance
Copper sulfate is commonly used in pool and spa maintenance to control algae growth and prevent cloudy water. It can be added directly to the water to kill algae and improve water clarity. Regular use of copper sulfate can help keep your pool or spa clean and inviting.
7. Ornamental Use
Copper sulfate is often used in horticulture as a coloring agent for floral arrangements. It can be used to dye flowers and foliage, adding vibrant colors to bouquets and other decorative displays. This can help enhance the visual appeal of floral arrangements.
8. Industrial Applications
In addition to its use in agriculture and horticulture, copper sulfate has various industrial applications. It can be used in electroplating, metal etching, and as a catalyst in chemical reactions. Its versatility and effectiveness make it a valuable component in various industries.
9. Affordable and Readily Available
Copper sulfate is a cost-effective solution for many applications. It is readily available in various forms, including powder, crystals, and solutions. Its affordability and accessibility make it a popular choice for both commercial and residential use.
10. Environmentally Friendly
When used properly and in the recommended doses, copper sulfate is considered environmentally friendly. It breaks down naturally in the environment and does not leave long-lasting residues. However, it should be used responsibly and according to guidelines to minimize any potential negative impacts.
Overall, copper sulfate offers numerous benefits across various industries and applications. Its effectiveness in weed control, algae control, fungal disease prevention, and other uses make it a valuable tool for maintaining healthy and productive environments.
Differences between Iron and Copper Sulfate
Iron and copper sulfate are two compounds that are commonly used in various applications. While both substances have similar properties, they also have some distinct differences. In this section, we will explore the contrasting characteristics of iron and copper sulfate.
1. Chemical Composition
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal that is widely used in industrial processes and everyday items. Copper sulfate, on the other hand, is a chemical compound with the formula CuSO4. It is a blue crystalline solid that contains copper, sulfur, and oxygen.
2. Physical Appearance
- Iron: Iron is a silver-gray metal that is known for its strength and durability. It can be found in various forms, including pure iron, alloys, and iron oxides.
- Copper Sulfate: Copper sulfate is a bright blue crystalline powder or granular solid. It is highly soluble in water.
3. Uses
- Iron: Iron is commonly used in the production of steel, which is vital for the construction industry. It is also used in the manufacturing of vehicles, appliances, and tools.
- Copper Sulfate: Copper sulfate has several uses, including agricultural applications as a fungicide and herbicide. It is also used in the electroplating industry and as a chemical reagent in various laboratory processes.
4. Reactivity
Iron is a less reactive metal compared to copper sulfate. It does not readily react with water or air, although it can rust when exposed to moisture and oxygen for extended periods.
Copper sulfate, on the other hand, is highly reactive. It can dissolve in water to form a blue solution and can also react with other chemicals to produce different compounds.
5. Toxicity
- Iron: Iron is an essential nutrient for humans and animals. However, excessive intake of iron can be toxic and may cause harmful effects on human health.
- Copper Sulfate: Copper sulfate is toxic and can cause adverse effects on humans and animals if ingested or inhaled in large amounts. It is important to handle it with care and use appropriate protective equipment.
6. Environmental Impact
Copper sulfate can have a negative impact on the environment if not used responsibly. It can contaminate water bodies and soil, leading to harm to aquatic life and plants. Iron, on the other hand, is less harmful to the environment.
Conclusion
In summary, iron and copper sulfate have distinct differences in terms of their chemical composition, physical appearance, uses, reactivity, toxicity, and environmental impact. While iron is widely used in various industries, copper sulfate has specific applications in agriculture and chemistry. Understanding these differences can help in determining the appropriate use of each compound.
When to Use Iron Sulfate
Iron sulfate, also known as ferrous sulfate, is a versatile and commonly used chemical compound. It is used in various applications due to its ability to provide iron to plants and treat iron deficiencies in soil. Here are some situations when iron sulfate can be used:
1. Treating Iron Deficiency
If your plants exhibit yellowing of leaves, known as chlorosis, it may indicate an iron deficiency. Iron sulfate can be used to treat this deficiency and improve plant health. It helps plants develop chlorophyll, which is responsible for the green color and aids in photosynthesis.
2. Correcting Soil pH Levels

If your soil has a high pH level, it can hinder the absorption of essential nutrients by plants. Iron sulfate can be used to lower soil pH levels, making the soil more acidic. This allows plants to unlock and absorb nutrients more effectively.
3. Moss Control
Iron sulfate is an effective moss control agent. It can be used to control moss growth in lawns, on roofs, or other areas where moss is unwanted. The iron in iron sulfate acts as a desiccant, drying out and killing the moss.
4. Algae Control in Water
In ponds, lakes, or water features where algae growth is a problem, iron sulfate can be used to control it. The iron in iron sulfate helps inhibit algae growth by interfering with its photosynthesis process. However, it is important to follow the recommended dosage and guidelines to avoid harming aquatic life.
5. Fertilizer for Acid-Loving Plants
Iron sulfate is a common ingredient in fertilizers for acid-loving plants such as azaleas, rhododendrons, and blueberries. These plants thrive in acidic soil, and iron sulfate provides both iron and acidity to promote healthy growth.
When using iron sulfate, it is important to follow the instructions and dosage recommendations. Overuse can lead to nutrient imbalances or pH extremes, which can harm plants. It is also recommended to conduct a soil test before application to ensure the correct dosage and need for iron sulfate.
When to Use Copper Sulfate
Copper sulfate is a versatile chemical compound that can be used for a variety of purposes. Here are some situations where you might consider using copper sulfate:
1. Algae and Weed Control
Copper sulfate can be an effective tool for controlling algae and weeds in ponds, lakes, and other bodies of water. It can be used to treat both filamentous algae, such as moss and string algae, as well as planktonic algae. Copper sulfate works by disrupting the algae’s ability to photosynthesize, leading to their death.
2. Fungicide
Copper sulfate is commonly used as a fungicide in agriculture to prevent and treat fungal infections on crops, fruits, and vegetables. It can be particularly effective against diseases like downy mildew, powdery mildew, and leaf spot.
3. Animal Repellent


Copper sulfate can be used as an animal repellent to deter pests like slugs, snails, and rats. Sprinkling copper sulfate granules around plants or creating a barrier with a copper sulfate solution can help protect your garden from unwanted visitors.
4. Cleaning and Sterilization
Copper sulfate can also be used as a cleaning agent and sterilizer. It has antimicrobial properties and can be used to disinfect tools, equipment, and surfaces. Copper sulfate can effectively kill bacteria, viruses, and fungi, making it a useful tool in maintaining cleanliness and preventing the spread of diseases.
5. Copper Deficiency in Plants
Copper sulfate can be applied to soil or sprayed on plants as a fertilizer to correct copper deficiencies. Copper is an essential micronutrient for plant growth, and its deficiency can lead to stunted growth and reduced crop yields. Applying copper sulfate can help ensure optimal plant health and productivity.
It is important to note that while copper sulfate can be a useful tool, it should be used carefully and in accordance with the product instructions. Overuse or misuse of copper sulfate can have negative impacts on the environment and aquatic life. It is always recommended to consult with a professional or follow the guidelines provided by the manufacturer when using copper sulfate.
Precautions and Considerations
When using iron or copper sulfate, there are several precautions and considerations that you should keep in mind to ensure safety and maximum effectiveness:
Protective Clothing

- Always wear protective clothing, such as gloves and goggles, when handling iron or copper sulfate.
- Avoid contact with skin, eyes, and clothing. In case of accidental contact, rinse the affected area immediately with plenty of water.
Proper Ventilation
- Use iron or copper sulfate in a well-ventilated area to prevent inhalation of fumes.
- If working indoors, ensure that the area is properly ventilated or use protective respiratory equipment.
Storage
- Store iron and copper sulfate in a cool, dry place away from direct sunlight.
- Keep them out of reach of children and animals.
- Do not mix iron and copper sulfate with other chemicals, as it may result in potentially dangerous reactions.
Environmental Impact
- Iron and copper sulfate can have harmful effects on aquatic life, so be cautious when using them near bodies of water or storm drains.
- Do not dispose of iron or copper sulfate in your sink, toilet, or any other drain. Follow local regulations for proper disposal methods.
Dosage and Application
- Always follow the recommended dosage and application instructions provided by the manufacturer.
- Use the appropriate concentration and quantity of iron or copper sulfate for the specific application.
- Avoid over-application, as it can lead to excessive build-up of iron or copper in the soil, which may be detrimental to plant health.
By following these precautions and considerations, you can safely and effectively use iron or copper sulfate for your intended purposes.
Question-answer:
What is iron sulfate?
Iron sulfate, also known as ferrous sulfate, is a chemical compound that contains iron in its +2 oxidation state. It is commonly used as a supplement in agricultural and gardening applications to correct iron deficiencies in plants.
What is copper sulfate?
Copper sulfate is a chemical compound that consists of copper in its +2 oxidation state. It is widely used as an agricultural fungicide and herbicide, as well as a disinfectant and algaecide in various industrial applications.
When should I use iron sulfate?
Iron sulfate is typically used when plants show symptoms of iron deficiency, such as yellowing leaves with green veins. It can be applied as a foliar spray or soil amendment to provide the necessary iron to plants and promote healthy growth.
When should I use copper sulfate?
Copper sulfate is commonly used in agriculture to control fungal and bacterial diseases on plants. It can also be used to kill algae and aquatic plants in ponds and water systems. Additionally, it is employed as a wood preservative and in various industrial processes.
Can I use iron sulfate instead of copper sulfate?
No, iron sulfate and copper sulfate have different uses and properties. While both compounds contain metals and can be used as nutrients for plants, they serve different purposes. Iron sulfate is used to correct iron deficiencies, while copper sulfate is primarily used as a fungicide, herbicide, and algaecide.
What are the potential risks of using copper sulfate?
While copper sulfate is effective for controlling pests and diseases, it can be toxic to aquatic life and should be used with caution near water sources. It can also be harmful if ingested or inhaled in large amounts. It is important to follow safety instructions and proper dosage when using copper sulfate.







