- Why Soil pH Levels are Important for Healthy Plant Growth
- The Role of pH in Nutrient Availability
- Effects on Microbial Activity
- Identifying Soil pH Levels
- Conclusion
- The Basics: What is Soil pH?
- Why is Soil pH Important?
- How is Soil pH Measured?
- Effects of pH on Nutrient Availability
- Managing Soil pH
- The Impact of Soil pH on Nutrient Availability
- Acidic Soil and Nutrient Availability
- Alkaline Soil and Nutrient Availability
- Optimal pH Range for Nutrient Availability
- The Role of Soil pH in Microbial Activity
- The Effects of pH on Plant Health and Disease
- Impact on Nutrient Availability
- Predisposition to Disease
- Root Development
- Leaf Color and Growth
- Conclusion
- Optimal pH Ranges for Different Plant Types
- 1. Acid-Loving Plants
- 2. Neutral pH Plants
- 3. Alkaline-Loving Plants
- Testing Soil pH: Methods and Tools
- 1. DIY pH Testing Kits
- 2. pH Meters
- 3. Laboratory Testing
- 4. Soil Testing Apps
- Adjusting Soil pH: Natural and Chemical Methods
- Natural Methods:
- Chemical Methods:
- “Question-Answer”
- Why is soil pH important for healthy plant growth?
- What is the ideal pH level for most plants?
- What happens if the soil pH is too acidic?
- How can I lower the pH of my soil if it is too alkaline?
- Is it possible to have different pH levels in different areas of my garden?
- Can I use vinegar to lower the pH of my soil?
- Can soil pH affect the color of plants?
- “Video” How does pH affect the Growth of Plants?
Have you ever wondered why some plants thrive while others wither away? The answer may lie in the pH level of the soil. Soil pH, or the measure of its acidity or alkalinity, plays a crucial role in determining the health and growth of plants. It affects the availability of nutrients in the soil, the activity of beneficial soil organisms, and the overall well-being of the plants.
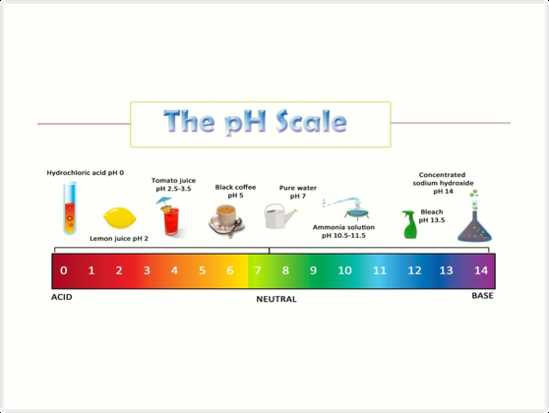
Soil pH is measured on a scale of 0 to 14, with 7 being neutral. Values below 7 indicate acidic soil, while values above 7 indicate alkaline soil. Most plants prefer a slightly acidic to neutral soil pH ranging from 6 to 7.5. However, different plants have different pH preferences, and understanding these preferences is key to successful gardening and farming.
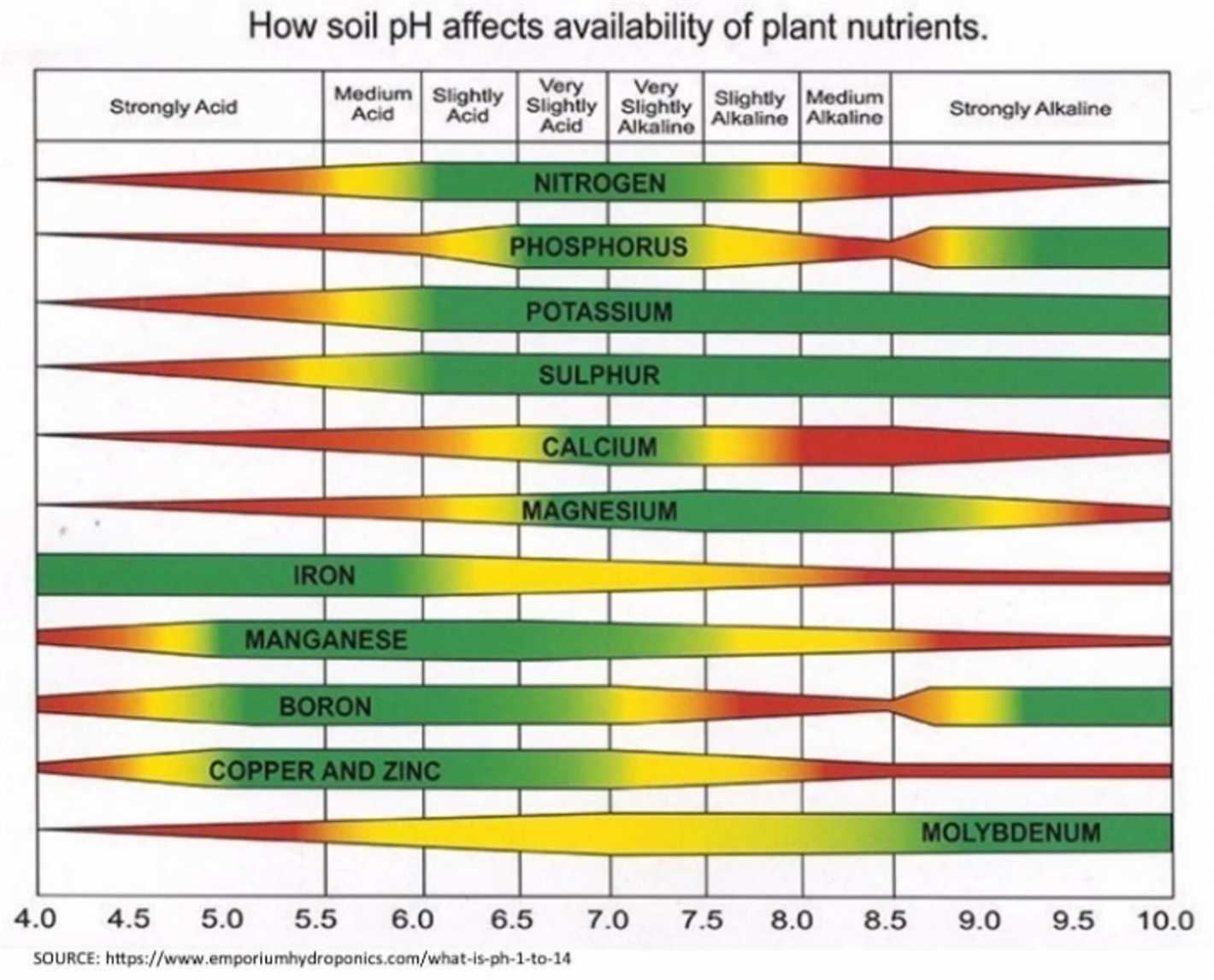
When the soil pH deviates from the ideal range for a specific plant, it can cause nutrient deficiencies or toxicities. For example, acidic soil with a low pH can hinder the availability of nutrients such as phosphorus, calcium, and magnesium. On the other hand, alkaline soil with a high pH can lead to an excess of certain nutrients, such as iron and manganese, which can be detrimental to plant growth.
It is essential to regularly test the pH of your soil and make adjustments if necessary to create an optimal growing environment for your plants. This can be done by using a soil pH testing kit or sending a soil sample to a laboratory for analysis. Once you know the pH level of your soil, you can make informed decisions about which plants to grow and how to amend the soil to meet their specific needs.
Why Soil pH Levels are Important for Healthy Plant Growth
A fundamental factor in promoting healthy plant growth is maintaining the appropriate soil pH levels. pH, a measurement of a substance’s acidity or alkalinity, can greatly affect a plant’s ability to absorb essential nutrients. Understanding the importance of soil pH levels is crucial for any gardener or farmer aiming to optimize their crop yields.
The Role of pH in Nutrient Availability
The pH level of soil directly impacts the availability of nutrients in the soil. Most plants require a slightly acidic to neutral pH range, typically between 6 and 7. When the soil pH deviates from this optimal range, it can hinder the plant’s ability to absorb essential elements such as nitrogen, phosphorus, and potassium.
For example, in soils with high acidity (lower pH), aluminum and manganese can become more soluble and toxic to plants, inhibiting their growth. On the other hand, in soils with high alkalinity (higher pH), nutrients like iron, zinc, and copper become less available to plants, leading to nutrient deficiencies.
Effects on Microbial Activity
Soil pH also influences the activity and diversity of beneficial soil microorganisms. Many microorganisms crucial for plant health thrive in specific pH ranges. These microorganisms help break down organic matter, fix nitrogen, and improve nutrient availability.
Changes in soil pH can disrupt the balance of these microorganisms, causing a decline in their activity. This can result in reduced nutrient cycling and availability, hampering plant growth and overall soil health.
Identifying Soil pH Levels
Testing soil pH is essential for understanding and managing its levels effectively. Home gardeners and farmers can use simple soil test kits or send samples to professional laboratories for more accurate analysis.
The results of soil pH tests can help determine the appropriate course of action, such as adding amendments to adjust the pH. For soils that are too acidic, agricultural lime or dolomite lime can be added to increase pH levels. To lower high pH levels, amendments like sulfur or organic matter can be incorporated into the soil.
Conclusion


When it comes to healthy plant growth, maintaining the appropriate soil pH levels is vital. The pH level affects nutrient availability, microbial activity, and overall plant health. By understanding the importance of soil pH and regularly testing and managing it, gardeners and farmers can ensure optimal growing conditions, leading to thriving plants and increased yields.
The Basics: What is Soil pH?
Soil pH refers to the level of acidity or alkalinity of the soil. It is measured on a scale of 0 to 14, with 7 being neutral. A pH below 7 indicates acidic soil, while a pH above 7 indicates alkaline or basic soil. The pH level of the soil plays a crucial role in the growth and development of plants.
Why is Soil pH Important?
The importance of soil pH lies in its influence on nutrient availability for plants. Different plants have different pH requirements for optimal growth. Some plants prefer acidic soil, while others thrive in alkaline conditions. If the soil pH is not suitable for a specific plant, it may struggle to take up essential nutrients, resulting in stunted growth or nutrient deficiencies.
Soil pH also affects the activity of microorganisms in the soil. Beneficial soil bacteria and fungi that assist in nutrient cycling and disease control have specific pH preferences. Maintaining the appropriate pH level can create a favorable environment for these organisms, leading to healthier plant growth.
How is Soil pH Measured?
Soil pH can be measured using a pH testing kit or by sending a soil sample to a laboratory for analysis. The testing kit typically includes pH test strips or a pH meter that measures the pH level directly. The laboratory analysis provides a more accurate and comprehensive assessment of the soil’s pH, along with other important soil parameters.
Effects of pH on Nutrient Availability
Soil pH affects nutrient availability by influencing the solubility and mobility of essential elements. Some nutrients, such as phosphorus, iron, and zinc, become less available to plants in alkaline soil. Acidic soil, on the other hand, may have increased availability of nutrients like manganese and aluminum, but excessive acidity can lead to toxicity issues.
The pH level also determines the effectiveness of soil amendments, such as lime and sulfur, in adjusting the pH. Adding lime can raise the pH of acidic soil, making it more suitable for plants that prefer a neutral or alkaline environment. Adding sulfur can lower the pH of alkaline soil, creating conditions that benefit acid-loving plants.
Managing Soil pH
Gardeners and farmers can manage soil pH by using appropriate amendments and cultural practices. Testing the soil regularly and adjusting the pH as needed ensures that plants have access to the necessary nutrients for healthy growth. It is important to consider the specific pH requirements of different plants and choose appropriate fertilizers and amendments accordingly.
| Plant | Preferred pH Range |
|---|---|
| Blueberries | 4.0-5.0 |
| Tomatoes | 5.5-6.8 |
| Lettuce | 6.0-7.0 |
| Potatoes | 5.0-6.0 |
By understanding the basics of soil pH and its importance, gardeners and farmers can create optimal growing conditions for their plants. Whether it’s adjusting the pH of the soil or choosing the right plants for existing soil conditions, maintaining the appropriate soil pH is essential for healthy plant growth.
The Impact of Soil pH on Nutrient Availability
The pH level of soil plays a crucial role in determining the availability of nutrients for plants. Different plants have different pH preferences, and the pH of the soil can greatly impact their ability to absorb essential nutrients.
Acidic Soil and Nutrient Availability
When soil is too acidic, with a pH below 6, certain nutrients become less available for plants. For example, phosphorus and potassium are less soluble in acidic soil, making it harder for plants to access these essential nutrients. Additionally, acidic soil can cause an accumulation of aluminum and manganese, which can be toxic to plants.
In acidic soil, the availability of nitrogen can also be affected. While some nitrogen compounds may become more soluble in acidic conditions, others can be lost through leaching. This can result in nitrogen deficiencies for plants, impacting their growth and overall health.
Alkaline Soil and Nutrient Availability
Conversely, when soil is too alkaline, with a pH above 7, it can also lead to nutrient deficiencies for plants. In alkaline soil, certain nutrients such as iron, zinc, and manganese become less available. This can particularly affect plants that require these nutrients for proper growth and development.
Alkaline soil can also affect the availability of phosphorus. In high pH conditions, phosphorus can become bound to other elements in the soil, making it less accessible for plants. This can lead to phosphorus deficiencies and hinder the plant’s ability to produce energy and carry out vital metabolic processes.
Optimal pH Range for Nutrient Availability
The optimal pH range for most plants is typically between 6 and 7. At this range, essential nutrients are more readily available for plant uptake. Nutrients are able to dissolve and be absorbed by plant roots more efficiently, supporting healthy growth and development.
It is important for gardeners and farmers to regularly test the pH level of their soil and make necessary adjustments to maintain optimal conditions for nutrient availability. This can include adding amendments such as lime to raise the pH of acidic soil or sulfur to lower the pH of alkaline soil.
| Nutrients | Availability in Acidic Soil | Availability in Alkaline Soil |
|---|---|---|
| Nitrogen | Can be lost through leaching | Becomes less available |
| Phosphorus | Becomes less soluble | Becomes bound to other elements |
| Potassium | Becomes less soluble | – |
| Iron, Zinc, Manganese | – | Become less available |
By understanding the impact of soil pH on nutrient availability, gardeners and farmers can make informed decisions when it comes to soil management and ensure optimal conditions for healthy plant growth.
The Role of Soil pH in Microbial Activity
Soil pH plays a crucial role in determining the activity of the microorganisms present in the soil. Microbial activity refers to the metabolic processes carried out by microorganisms such as bacteria, fungi, and actinomycetes, which are responsible for the decomposition of organic matter, nutrient cycling, and natural soil processes.
Effect of pH on Microbial Activity:
The pH of the soil affects the growth and activity of microorganisms in several ways:
- Optimum pH: Each group of microorganisms has an optimal pH range at which they thrive. For example, bacteria and actinomycetes prefer neutral to slightly acidic soil conditions (pH 6-7), while fungi prefer slightly acidic to slightly alkaline conditions (pH 5-8). When the soil pH deviates from their optimum range, the activity of these microorganisms may be significantly reduced.
- Enzyme Activity: Microorganisms produce enzymes that play a vital role in breaking down organic matter and making it available for plant uptake. The activity of these enzymes is influenced by soil pH. In acidic soil conditions, enzyme activity may be limited, resulting in a slower decomposition process.
- Nutrient Availability: Soil pH affects the availability of essential nutrients for microbial growth. Some nutrients, such as phosphorus, iron, and manganese, are less available in alkaline soil conditions, while others, such as nitrogen and potassium, are less available in acidic soil conditions. The availability of these nutrients impacts microbial activity, as they require a sufficient nutrient supply to carry out their metabolic processes.
Implications for Plant Growth:
The activity of microorganisms in the soil directly impacts plant growth and health. Microorganisms aid in the decomposition of organic matter, releasing essential nutrients for plant uptake. They also help improve soil structure and enhance water retention capacity. When soil pH is outside the optimal range for microbial activity, nutrient availability and decomposition processes may be compromised, affecting plant growth and overall soil health.
Managing Soil pH for Microbial Activity:
To optimize microbial activity in the soil, it is essential to manage soil pH within the preferred range for different groups of microorganisms. This can be achieved through the following measures:
- Conducting soil tests to determine the pH of the soil and identify any necessary amendments.
- Adding organic matter, such as compost or manure, to the soil to improve its pH and nutrient content.
- Using lime or sulfur-based amendments to raise or lower pH, respectively, based on the specific needs of the plants being grown.
- Regularly monitoring and adjusting soil pH to maintain optimal conditions for microbial activity.
By understanding the role of soil pH in microbial activity and taking appropriate measures to manage it, gardeners and farmers can create a favorable soil environment that promotes healthy plant growth and maximizes the benefits provided by microorganisms.
The Effects of pH on Plant Health and Disease
The pH level of soil plays a crucial role in the health and disease resistance of plants. The pH scale ranges from 0 to 14, with 7 considered neutral. pH levels below 7 are acidic, while levels above 7 are alkaline. Different plants have different preferences for pH levels, and when the soil pH deviates from their ideal range, it can lead to various issues.
Impact on Nutrient Availability

The pH level of soil directly affects the availability of nutrients to plants. Some nutrients, such as nitrogen, phosphorus, and potassium, are more readily available in slightly acidic soils, with a pH between 6 and 6.5. On the other hand, alkaline soil with a pH above 7 can inhibit the absorption of essential nutrients, making them less available to plants. This imbalance in nutrient availability can lead to nutrient deficiencies or toxicities, affecting the overall health and growth of plants.
Predisposition to Disease
Soil pH also influences the susceptibility of plants to diseases. Certain plant pathogens thrive in specific pH ranges. For example, some fungal diseases, like root rot, prefer acidic soil conditions, while others may thrive in alkaline soils. When the pH level is not suitable for a particular plant, it weakens the plant’s immune system, making it more vulnerable to infections and diseases.
Root Development
The pH level of soil significantly impacts root development. When the soil pH is too extreme, either too acidic or too alkaline, it can hinder root growth. Roots absorb water and nutrients from the soil, and they need an optimal pH level to function efficiently. If the pH is too high or too low, roots may struggle to absorb nutrients properly, leading to stunted growth and overall poor plant health.
Leaf Color and Growth
The pH level of soil can affect the color and growth of plant leaves. In some cases, a change in soil pH can cause nutrient imbalances and lead to chlorosis, a condition where the leaves turn yellow due to a lack of chlorophyll. Chlorosis can weaken plants and make them more susceptible to diseases and pests. Additionally, plants may experience stunted growth and have difficulty producing vibrant and healthy foliage if the pH is not within their ideal range.
Conclusion
The pH level of soil is a crucial factor in determining the health and disease resistance of plants. By understanding the impact of pH on nutrient availability, disease susceptibility, root development, and leaf color and growth, gardeners and farmers can take appropriate measures to maintain the optimal pH levels for their desired plant species. Regular soil testing and proper soil amendments can help ensure that plants receive the necessary conditions for healthy growth and productivity.
Optimal pH Ranges for Different Plant Types
When it comes to the growth and development of plants, the pH level of the soil plays a crucial role. Different plant types thrive in different pH ranges, and understanding these optimal ranges can help gardeners and farmers achieve better yields and healthier plants. Here are some common plant types and their preferred pH ranges:
1. Acid-Loving Plants
Examples: Blueberries, azaleas, rhododendrons
Optimal pH Range: 4.5 to 5.5
Acid-loving plants prefer acidic soil conditions. Maintaining a pH level within the optimal range helps these plants access nutrients and promotes proper root development. To adjust the pH for acid-loving plants, gardeners can use amendments such as peat moss, sulfur, or iron sulfate.
2. Neutral pH Plants
Examples: Most vegetables, flowers, and grasses
Optimal pH Range: 6.0 to 7.0
Many common plants, including vegetables, flowers, and grasses, thrive in neutral pH conditions. These plants can access nutrients efficiently and establish healthy root systems when the soil pH is within the optimal range. Adding organic matter like compost or well-rotted manure can help maintain the pH balance.
3. Alkaline-Loving Plants
Examples: Lilacs, lavender, forsythia
Optimal pH Range: 7.0 to 8.0
Some plants, like lilacs and lavender, prefer alkaline soil conditions. In such alkaline conditions, these plants can efficiently uptake nutrients and thrive. To increase the alkalinity of the soil, gardeners can add materials like limestone or wood ash.
It is important to note that while specific plants have preferred pH ranges, they can tolerate slight variations. Regularly monitoring the pH level of the soil and making necessary adjustments will ensure optimal growing conditions for the plants you are cultivating.
Here is a summary of the optimal pH ranges for different plant types:
| Plant Type | Optimal pH Range |
|---|---|
| Acid-Loving Plants | 4.5 to 5.5 |
| Neutral pH Plants | 6.0 to 7.0 |
| Alkaline-Loving Plants | 7.0 to 8.0 |
Testing Soil pH: Methods and Tools
Testing soil pH is an essential step in understanding the health and fertility of your soil. Depending on the results, you can make necessary adjustments to ensure optimal plant growth. There are several methods and tools available for testing soil pH.
1. DIY pH Testing Kits
One of the most common and convenient methods for testing soil pH is by using DIY pH testing kits. These kits typically include a set of color-coded strips or tablets that change color based on the pH level of the soil. To use the kit, you need to extract a small amount of soil and mix it with water according to the kit’s instructions. Then, you dip the strips or add the tablets to the soil-water mixture and compare the resulting color with the kit’s color chart to determine the pH level.
2. pH Meters
pH meters are electronic devices that provide a quick and accurate measurement of soil pH. These meters consist of a probe or electrode that is inserted directly into the soil. The electrode measures the electrical potential of the soil and converts it into a pH value. pH meters are relatively simple to use, but they require regular calibration to ensure accurate readings.
3. Laboratory Testing
For a comprehensive analysis of soil pH, you may consider sending a soil sample to a laboratory for professional testing. This method provides the most accurate and detailed results as it can measure other soil properties apart from pH. Many agricultural extension offices and private testing labs offer soil testing services. You will need to collect a representative soil sample from your garden or field and follow the specific instructions provided by the laboratory for sample collection and submission.
4. Soil Testing Apps
In recent years, smartphone apps have emerged as a convenient tool for soil testing. These apps use the phone’s camera to capture an image of the soil sample and analyze it using algorithms and machine learning. The apps can provide instant results and recommendations based on the soil pH and other soil attributes such as nutrient levels and organic matter content. While they may not be as accurate as laboratory testing, they can be a useful tool for quick assessments.
| Method | Pros | Cons |
|---|---|---|
| DIY pH Testing Kits | – Easy to use – Inexpensive | – Less accurate than other methods – Limited information |
| pH Meters | – Quick results – Accurate readings | – Requires regular calibration – More expensive |
| Laboratory Testing | – Comprehensive analysis – Accurate results | – More expensive – Longer waiting time |
| Soil Testing Apps | – Convenient and quick – Provides recommendations | – Less accurate than laboratory testing – Relies on algorithms |
Choosing the appropriate method for testing soil pH depends on your needs, budget, and the level of accuracy required. Regardless of the method you choose, regularly testing your soil pH is crucial for maintaining healthy plant growth and maximizing crop yields.
Adjusting Soil pH: Natural and Chemical Methods
Once you have determined the pH level of your soil and identified the ideal range for your plants, it may be necessary to adjust the pH to create a more favorable growing environment. There are two main methods for adjusting soil pH: natural methods and chemical methods.
Natural Methods:
- Adding organic matter: Incorporating organic matter into the soil, such as compost or well-rotted manure, can help buffer the pH and make it more stable over time.
- Using amendments: Certain amendments, like sulfur or aluminum sulfate, can be added to the soil to lower the pH. These materials react with the alkaline compounds, releasing ions that result in an overall lower pH.
- Liming: On the other hand, if you need to raise the pH of your soil, agricultural lime can be used. Lime is made up of calcium or magnesium compounds that neutralize acidic compounds, making the soil more alkaline.
Chemical Methods:
- Acidic fertilizers: Fertilizers that have an acidic pH can be applied to lower the soil pH. These fertilizers contain compounds like ammonium, which releases ions that reduce the alkalinity of the soil.
- Liquid additives: There are liquid additives available on the market designed specifically to adjust soil pH. These additives can be mixed with water and applied directly to the soil.
- pH testing kits: pH testing kits can be used to monitor the pH levels in your soil regularly. By testing the soil regularly, you can make any necessary adjustments before they become major issues.
When adjusting soil pH, it is important to keep in mind that changes may take time. It is best to make small adjustments and retest the soil periodically to ensure you are moving towards the desired pH range. Additionally, it is important to follow the instructions provided by the manufacturer when using any chemical methods to adjust soil pH, as excessive use can lead to negative impacts on plant health.
“Question-Answer”
Why is soil pH important for healthy plant growth?
Soil pH is important for healthy plant growth because it affects nutrient availability, microbial activity, and the overall health of the soil. Different plants have different pH preferences, so maintaining the appropriate pH level can ensure that plants have access to the nutrients they need to thrive.
What is the ideal pH level for most plants?
The ideal pH level for most plants is between 6 and 7. This slightly acidic to neutral pH range allows for optimal nutrient availability in the soil. Some plants have specific pH preferences outside of this range, so it’s important to research the specific pH requirements of the plants you are growing.
What happens if the soil pH is too acidic?
If the soil pH is too acidic, certain essential nutrients like nitrogen, phosphorus, and potassium may become less available to plants. This can lead to nutrient deficiencies and poor plant growth. Additionally, acidic soil can also harm beneficial soil microorganisms that help with nutrient cycling and plant health.
How can I lower the pH of my soil if it is too alkaline?
If your soil is too alkaline, you can lower the pH by adding amendments such as elemental sulfur, aluminum sulfate, or peat moss. These substances can help to acidify the soil and bring the pH down to a more suitable range for your plants.
Is it possible to have different pH levels in different areas of my garden?
Yes, it is possible to have different pH levels in different areas of your garden. This can happen due to variations in soil composition, organic matter content, and previous fertilizer applications. It’s important to test the pH of your soil in various areas of your garden to ensure that you are providing the ideal conditions for your plants.
Can I use vinegar to lower the pH of my soil?
Yes, vinegar can be used to lower the pH of your soil, but it should be used with caution. Vinegar is a strong acid and can cause harm to both plants and beneficial soil organisms if used in excessive amounts. It’s best to use vinegar in small quantities and monitor the pH carefully to avoid over-acidifying the soil.
Can soil pH affect the color of plants?
Yes, soil pH can affect the color of certain plants. For example, hydrangeas can change color based on the pH of the soil. In acidic soil (pH below 6), hydrangeas tend to be blue, while in alkaline soil (pH above 7), they tend to be pink. Adjusting the pH of the soil can therefore be used as a way to manipulate the color of certain flowers.







